Patrick Ryan, NIH Trainee 2021-2023
Patrick Ryan, NIH Trainee 2021-2023
Graduate Program: Molecular and Cellular Biology
Lab: Jungwoo Lee
Research Interests: 3D in vitro models to study bone cancer metastasis
Research Summary
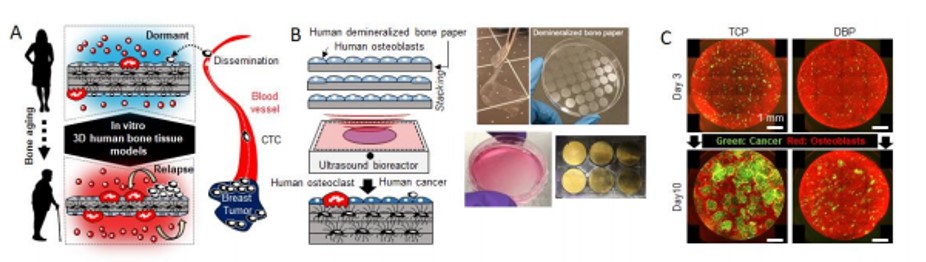
Metastatic relapse of disseminated tumor cells (DTC) after years of dormancy on the bone is the leading cause of death in many prominent cancers but remains poorly understood due to a lack of clinically relevant models. Tumor cell dissemination on the surface of bone occurs in early stage cancer but DTCs lie dormant until their surrounding microenvironments become favorable for their regrowth. Compelling evidence exists that bone cancer metastasis (BCM) is functionally linked to bone remodeling as metastatic bone tumor always accompanies osteolytic or osteoblastic lesions. Advancing aging is the most critical risk factor for metastasis. It is critical to understand the mechanism behind why DTCs become dormant in healthy bone and awaken in aged bone. The goal of my dissertation is to create bioengineered human bone tissue models and apply this model to dissect the role of aging bone microenvironment in DTC relapse.
First, I will build 3D human bone tissue models by additively layering human osteoblast-seeded demineralized bone paper (DBP) . DBP developed in our lab preserves intrinsic bone extracellular matrix complexity, directs osteoblasts to restore in vivo-relevant phenotype, and allows easy experimental handling and microscope imaging1. A key difference between young and old bone is its thickness. This layered approach allows us to build different thickness of bone tissue models. We hypothesize that young and old bone tissue microenvironments can be reproduced by adjusting the thickness of 3D human bone tissue models. I will characterize the thickness dependent soluble factor secretion and response to chemical stimulants. By establishing young and old bone tissue models, I can begin to systematically investigate the role of bone tissue aging and BCM.
Next, I will inoculate human breast/prostate cancer cells to simulate when DTCs land on the surface of bone. Total area imaging and time-lapse imaging will be conducted during the co-culture period. I expect this model can reproduce cellular and mass dormancy. Dormant and active state tumor cells will be confirmed by P21 and Ki67 antibody staining. By establishing dormant metastasis models, I can begin to study how dormant tumor cells re-awaken.
Finally, I will study the role of bone remodeling process in promoting dormant tumor cells growth. I will culture CD14 monocytes derived from human peripheral blood in the presence of RANKL and M-CSF to induce osteoclast differentiation. We postulate that decreasing bone mass reduces the ability to control localized bone remodeling and extended remodeling period and area will promote tumor cell growth. Mineral resorption and collagen degradation will be monitored by fluorescent calcein and pro-Collagen ELISA. I will conduct multiplex immunofluorescent staining to determine whether bone remodeling activity has functional connection with tumor cell growth. We will also investigate the resting and active state of osteoblasts in directing osteoclastogenesis and related tumor cell growth. This study will provide new insight into how these clinically used drugs modulate bone metabolism and suppress BCM that could contribute to identifying new therapeutic targets.
Established preclinical 3D human bone tissue models represent great potential to improve predictive power of preclinical studies and understand the working mechanism of anti-metastasis drugs such as zoledronate and denosumab. We expect this platform technology will accelerate high-throughput anti-BCM drug discovery.
Figure 1. Development of 3D in vitro human bone tissue models to study bone metastasis. (A) The need to develop human bone tissue models (B) Bioengineering approach to create 3D human bone tissue models. 6 Layer stacked DBP seeded with human osteoblasts (bottom left). 6 well plate ultrasonic mechano-culture device (bottom right). (C) Representative results of human cancer growth between TCP and DBP in co-culture with dsred osteoblasts.