Jacob Ullom, NIH Trainee 2021-2023
Jacob Ullom, NIH Trainee 2021-2023
Graduate Program: Molecular and Cellular Biology
Lab: Leonid Pobezinsky
Research Interests: Understand the mechanism by which T cells of the adaptive immune system differentiate into either terminal effectors or memory cells upon activation.
Research Summary
The immune system is the main barrier which protects our bodies from disease. One of the primary parts of our immune system is the adaptive immune system, which responds to specific antigens. This specificity is derived from the cell types which comprise adaptive immunity, one of the most important cell types being T cells. T cells come in two types: CD4 helper cells and CD8 killer cells. Helper CD4 T cells regulate the immune response through secretion of signaling proteins called cytokines, while killer CD8 T cells destroy infected cells via direct contact and secretion of cytotoxic granules. To perform their function, T cells must first be activated through contact between their T cell receptors and a displayed peptide antigen originating from a foreign target. Once activated, T cells have two distinct cell types or “fates” which they can differentiate into: terminal effectors or memory cells.
Terminal effectors are highly active T cells which arise from chronic stimulation of their T cell receptors. While terminal effectors are active, they also have a high number of inhibitory receptors on their surface. When stimulated, these inhibitory receptors can disrupt the activation signal, causing a loss of killing ability and eventually cell death. Memory cells are the opposite of terminal effectors as when activated, they become dormant rather than killers. Once restimulated by returning antigen, memory cells gain efficient killing ability and quickly clear the returning foreign attackers. Memory cells also express low amounts of inhibitory receptors on their surface, even when restimulated. Certain types of tumors will use terminal effectors’ high number of inhibitory receptors to their advantage by expressing higher than normal amounts of inhibitory ligands on their surface, preventing an effective T cell response in the tumor microenvironment. It has been shown that restimulated memory cells are able to overcome the inhibitory tumor microenvironment due to the lack of inhibitory receptors on their surface. The Pobezinsky lab is currently studying the molecular mechanism of T cell differentiation with a focus on killer CD8 T cells.
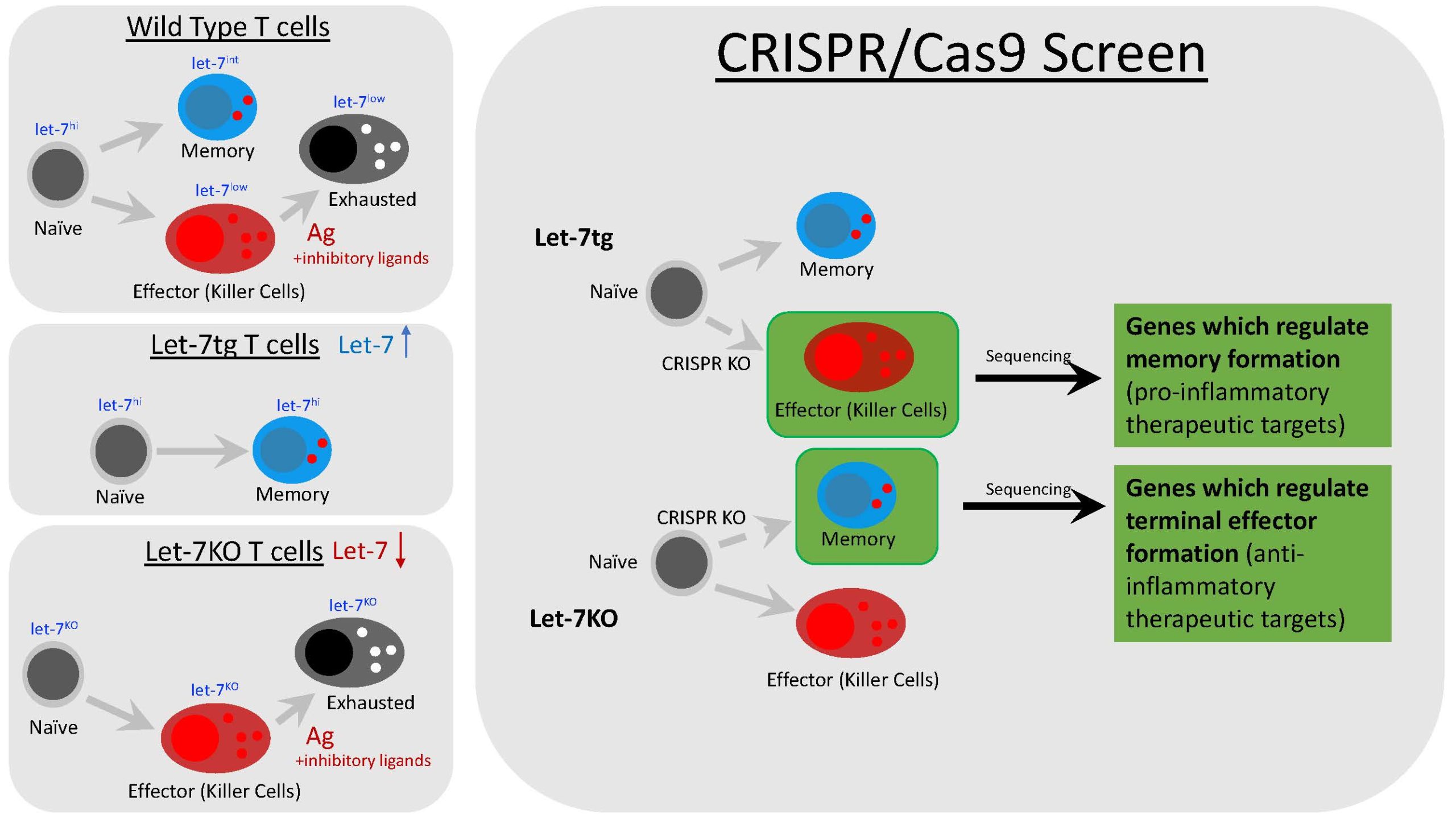
The lab’s original focus was to discover how a microRNA called lethal-7, or let-7, controlled T cell activity. To this end, mouse model systems were made: one type which is let-7 deficient, and another which overexpresses let-7 through a transgene. Upon examination of killer CD8 T cells, it was found that let-7 controls T cell differentiation, as let-7 deficient cells exclusively became terminal effectors and let-7 transgenic cells exclusively became memory. This exciting discovery shifted our focus to understanding more about T cell differentiation using this model system.
My current project is focused on developing a screen to find and catalog genes which promotes either terminal effector or memory formation in cytotoxic CD8 T cells, using our let-7 model system. We will use CRISPR/Cas9 technology to knock-out a library of every single protein coding gene in CD8 killer T cells from either let-7 transgenic or let-7 deficient mice. Once the screen is complete, genes which control memory formation, terminal effector formation, or both will be revealed through deviation of differentiation of our model. For example, let-7 transgenic cells differentiate into terminal effectors due to a knock-out of a gene important for memory formation. From here, we can not only identify novel therapeutic targets, but also discover new pathways which regulate the formation of terminal and memory cells.