Jiacheng Wang, UMass Fellow 2021-2023
Jiacheng Wang, UMass Fellow 2021-2023
Graduate Program: Chemical Engineering
Lab: Michael Henson
Research Interests: Investigating the impact of public versus private metabolism on the stability of microbial communities within natural hosts
Research Summary
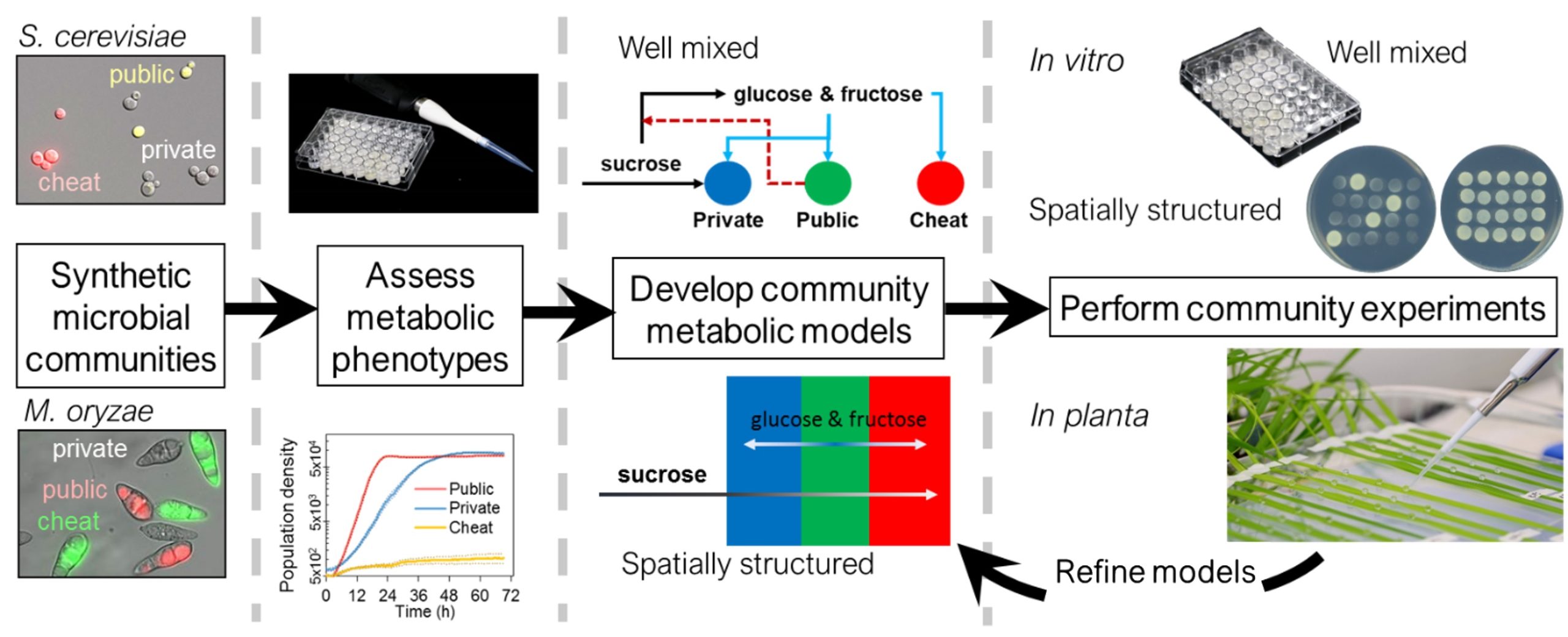
Microorganisms do not exist in isolation; instead, they form intricate communities of diverse strains where individuals participate in complex cooperative and competitive interactions. Despite the importance of these communities in natural ecosystems and engineered bioprocesses, we lack a comprehensive understanding of how these interactions alter community function and stability that are crucial for predicting the evolution of microbial strategies that promote survival and growth.
To survive and thrive, microbes must obtain nutrients from their environment through cooperative and competitive actions. A common strategy to obtain nutrients involves secreting enzymes into the external, “public” environment to break down or capture resources before they are taken up into the cell. The metabolic products are cooperative public goods as they are generated externally and so benefit other cells in the shared environment. This seemingly successful strategy termed “public metabolism” is used by a wide range of microbial species that inhabit diverse habitats, yet it has two obvious drawbacks. First, the public goods can easily be lost into the environment before they are successfully taken up by the cell that generated them. Second, the public goods can be exploited by microbes not contributing to their production but still reap the rewards. These shortcomings can threaten the success of public metabolism and the stability of microbial communities. An exploitation-free strategy exists whereby microbes can secure nutrients by taking them directly into the cell, with digestion taking place “privately” inside the cell, instead of “publicly” in the environment. Yet despite this seemingly preferable alternative, many microbes still feed by public metabolism.
Based on preliminary data generated by our experimental collaborators, we hypothesize that sufficiently spatially structured environments will limit the exploitation of public metabolizers, thus favoring them over private metabolizers that dominate in planktonic cultures. To test this hypothesis, we have developed two well-defined and tractable synthetic yeast communities to probe the fitness of different metabolic strategies experimentally and computationally in their natural environments and assess community stability and function. The research will enable us to extrapolate general principles from system-specific observations and to develop a classification of different types of biotic (e.g., host-pathogen and microbe-microbe) and abiotic (e.g., spatial structure) conditions that favor cooperative, public metabolism.