 Francesca Anson Francesca AnsonProgram: Chemistry Advisors: Jeanne Hardy, PhD. and Sankaran Thayumanavan, PhD. Education: UC Riverside, BS, Chemistry, 2013 |
Research Summary
Characterize and expand the formulation of potent apoptosis inducing nanogels using combinations of enzymes and small molecule therapeutics for targeted delivery
Exploiting apoptosis is a powerful and convenient way to trigger selective death of cancerous cells. The goal of my project is to develop delivery materials that are capable of selectively killing subpopulations of undesirable cells. The delivery of apoptosis inducing agents, 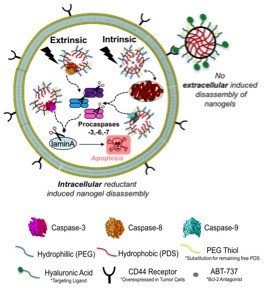 such as cytotoxic small molecules and anti-mitotic drugs, to cancer cells have been successful but often results in non-specific, off-target delivery and activity in neighboring healthy cells. Simultaneously delivering small molecule apoptosis inducing agents with apoptosis inducing proteins will demonstrate an interdisciplinary approach to synergistically provoke and induce cell suicide. The ability to selectively target cells so that hi
such as cytotoxic small molecules and anti-mitotic drugs, to cancer cells have been successful but often results in non-specific, off-target delivery and activity in neighboring healthy cells. Simultaneously delivering small molecule apoptosis inducing agents with apoptosis inducing proteins will demonstrate an interdisciplinary approach to synergistically provoke and induce cell suicide. The ability to selectively target cells so that hi
gh concentrations of apoptosis inducers are present at the tumor site and low amounts accumulate in healthy neighboring cells is of great interest. Polymeric nanoparticles introduce a robust framework for therapeutic delivery vehicles capable of delivering small molecules as well as fragile proteins. Utilizing polymeric nanoparticles for small molecule delivery avoids, or attempts to minimize the undesirable affects mentioned.
The Thayumanavan group has established a tunable, stimuli responsive nanogel delivery vehicle composed of a polyethylene glycol and pyridyldisulfide polymer. The nanogel delivery vehicles can be tuned to improve their biocompatibility, functionalized for cell-specific uptake, and crosslinked to different degrees to improve cargo stability and control the rate of cargo release – small molecule or protein. In the Hardy group, we focus on understanding cysteine aspartate proteases (caspases), executioner or initiator, from a molecular level to utilize their apoptotic role and function so that they may be targeted therapeutically. As a joint student between the two labs, I plan to build on our nanogel-caspase technology and target different steps within the extrinsic apoptotic pathway. This will allow us to develop natural delivery systems capable of executing selective killing in a number of diseases beyond cancer, such as viral infections. To monitor and compare the conjugates uptake in vitro, visualize their localization and understand their mechanism of uptake and cell death, I will engineer both the polymer and various caspases to contain specific fluorescent signatures. Characterizing the specific conjugate signals collaboratively with the Department of Chemical Engineering and Physics will give us more information on the fundamentals influencing their self-assembly. These collaborations combined with my role as a liaison between the Hardy and Thayumanvan groups, empower additional collaboration for myself and other members of all groups. This dynamic easily facilitates the creation of professional relationships, the direct dispersion of chemical, physical, and biochemical knowledge regularly as well as the extension for all to different instrumentation equipment and characterization methods. I foresee my interdisciplinary comprehension in organic nanogel formulations, biochemical caspase manipulation and academia to industry transitions to be crucial in successfully formulating and characterizing potent apoptosis inducing nanogels to be used as biotechnological therapeutics.
