 The biotech community at UMass has a decade-long tradition that started as part of the Institute Cellular Engineering to teach laboratory modules on cutting edge research techniques.
The biotech community at UMass has a decade-long tradition that started as part of the Institute Cellular Engineering to teach laboratory modules on cutting edge research techniques.
These modules will continue under BTP and can be attended by industrial partners interested in learning new methods and using UMass facilities. Laboratory modules are held at various locations on the UMass campus, sometimes during winter break, spring break and during the summer.
42 Labs Modules offered since BTP inception and taught to date
3-D printing for Biotechnology Applications
Anaerobic Systems Microbiology
Analysis of DNA Methylation Using Pyrosequencing
Assessment of Macrophage Repolarization using Flow Cytometry
Automating Plant Transformations
Bioimaging
Bioreactors and Protein Analysis
Bone Marrow Hematopoietic Colony Forming Assay
Building a Microscope
Characterization of Bioconjugates and other Materials by Mass Spectrometry (MS)
CRISPR in IPS Cells
Designing & Building Fluidic Devices for Biomedical Research
DNA/RNA Nanotechnology & Aptamers
Drug Delivery
Fundamentals of AMNIS: Imaging Flow Cytometry
Glass Blowing
Hydrogen-deuterium Exchange Mass Spectrometry
Intro to Fermentation and High-Throughput Screening
Introduction to 3-D Printing
Introduction to Biomaterials and their Applications
Introduction to Cell Culture
Introduction to Genome Expression Analysis
Introduction to Synthetic Biology
IPython and the Systems Biology Knowledgebase (KBase)
Kinesin Motor Protein Purification and Biophysical Assessment
Live-cell Quantitative Flurorescence Microscopy
Modeling Cellular Metabolism and Processes
Molecular Modeling and Simulation Using Computational Approaches
Monoclonal Antibody Production
Multi-Color Total Internal Reflection Fluorescence and Super-Resolution Imaging
NMR Spectroscopy
Optical Trapping
Patch Clamp Electrophysiology
Polymer Analysis and Characterization: Applications for Engineered Biomaterials
Polymerase Chain Reaction (PCR)
Quantitative Fluorescence Microscopy and Image Analysis
Quantitative Reconstruction of Three-Dimensional Fluorescence Images
Signal Multiplexing with the MAGPix Platform
Skills for Scientific Communication
Structural Characterization of Biomaterials using Scattering
Topics on Fluorescence Spectroscopy: Fluorescence Lifetime Determinations
UMass Amherst Software Carpentry for Unix, Git, and R
Vitrobot CryoEM Module
Zebrafish Toxicity Assays
A list of prior laboratory modules can be found here.
Below is are more detailed descriptions of the many lab modules offered:
3-D printing for Biotechnology Applications.
This course is designed for graduate students who are new to 3D printing or who would like to learn more about 3D printing. The goal of the course is to use 3D printing to create a part or tool that benefits your research. Students will use the equipment available at the Advanced Digital Design and Fabrication Core Facility in the Life Sciences Lab building. BTP faculty who have worked with ADDFab in the past will share examples of how they have designed parts for their research. The core facility director will explain the various types of 3D printing and how to choose the appropriate technology. Students will be expected to come up with their own idea for a device or tool and will be paired with a CAD (computer aided design) mentor who will assist the student in generating a computer model and printing a first-generation prototype. Then, the student will test out the prototype in the lab and repot to class how well it worked. Everyone will make changes to their designs and print a 2nd generation part. At the end of the course, each student will present a summary of how the performance of their 1st generation device led to design changes in their 2nd generation device and impacted the function of the function of the 2nd generation device.
This course is designed for graduate students who are new to glass blowing or who would like to learn more about scientific glass blowing. The goal of the course is to use glass blowing to create a part or tool that benefits your research. Students will use the equipment available in the Glass Blowing Core Facility workshop. The core facility director will explain the various types of glassblowing. Students will have the opportunity to build their own projects.
Prof. Changhui Pak and Mike Daley, the director of the UMass Cell Culture Facility are offering an exciting course will serve as a primer for advanced graduate students who want to incorporate human iPSC research and/or CRISPR mediated gene engineering. Students will learn the basic knowledge of CRISPR systems, work with mammalian cells and iPSCs and engage in discussions on how to design specific experiments using CRISPR/iPSC tailored to their ongoing research projects. Module in the format of a combination of hands-on lab time and short lectures will be used..
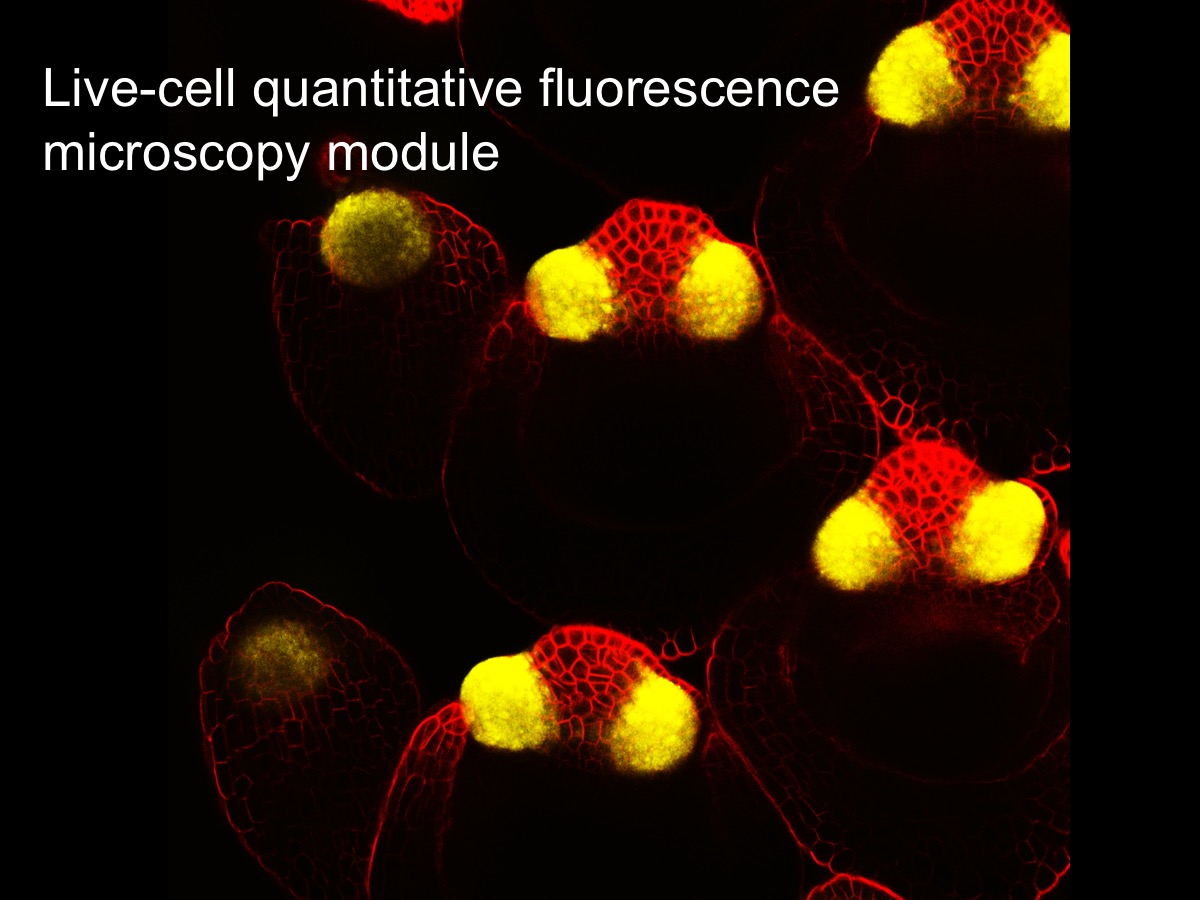
Live-cell quantitative Fluorescence microscopy:
This lab module will introduce fundamental concepts of fluorescence microscopy using living tissue culture cells. After learning about the principles, strengths, and weaknesses of different approaches, participants will apply and compare wide-field fluorescence imaging, spinning disk and point scanning confocal imaging, and structured illumination microscopy (SIM) super-resolution imaging – all on living samples. Participants will learn how to control multiple microscopy systems (hardware) using the NIS-Elements software platform. The training will also include quantitative image analysis of data acquired in the module.
Instructor(s): Tom Maresca, Biology Associate Professor and James Chambers, Director of Light Microscopy Core Facility
Introduction to 3D Printing:
3D bioprinting of cellularized constructs has enabled the reconstruction of organs, revascularization of blood vessels, and other tissue engineering applications that require complex geometries. Traditional FDM 3D printing is limited by the presence of support materials that restrict the design process. This BTP module will cover the limitations and solutions to FDM printing that enable bioprinting of complex structures not restricted to this degree of freedom. The training will introduce FDM 3D printing, via hands-on CAD and plastic printing, and culminate in bioprinting with alginate and collagen constructs, which will begin with a material preparations module.
Instructors: Katharine Bittner, Duy Nguyen, Hyuna Kim
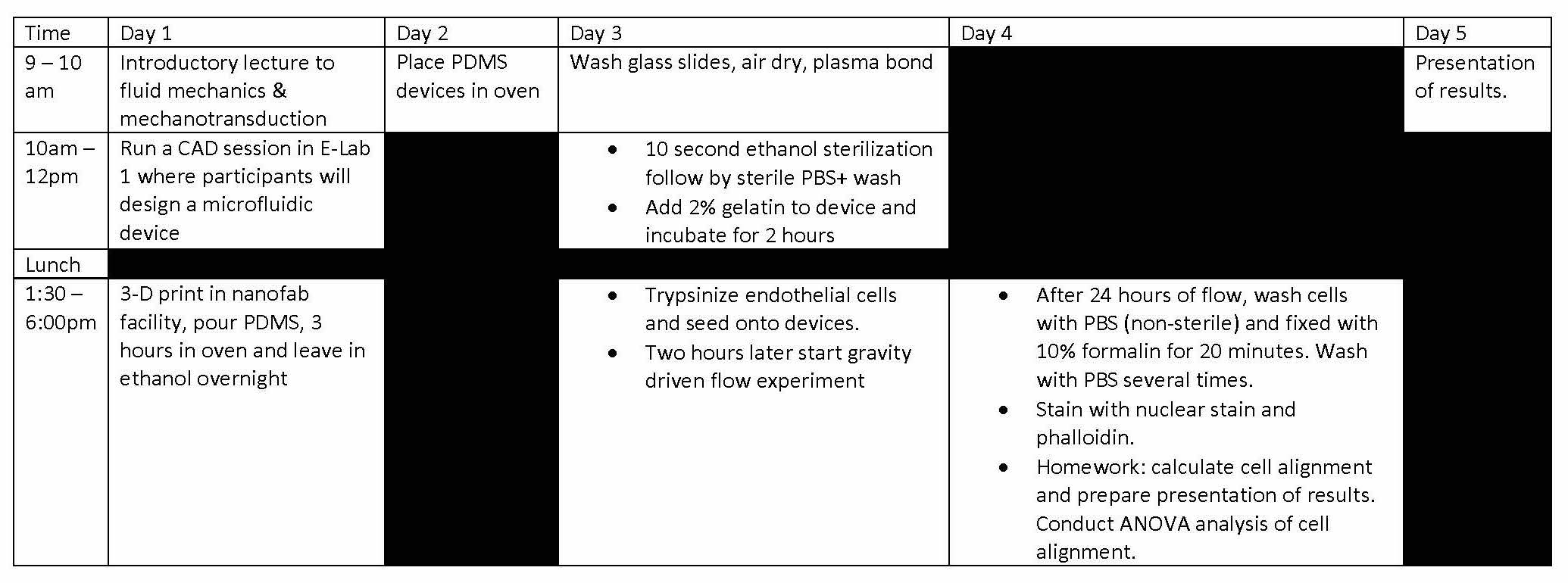
Designing & Building Fluidic Devices for Biomedical Research
Microfluidic devices have become popular commercial on-site medical diagnostic tests and in the research realm have played an important role in elucidating biological mechanisms. This module will use microfluidic devices to introduce participants to the basics of mechanotransduction in biology and medicine. this module will cover basic topics in vascular biology and fluid dynamics. Students will design and develop fluidic devices that can be used for flowing blood, growing cells in culture and expose them to fluid forces mimicking mechanical cues experienced in vivo.
Instructor: Dr. Juan Jimenez (Mechanical and Industrial Engineering)
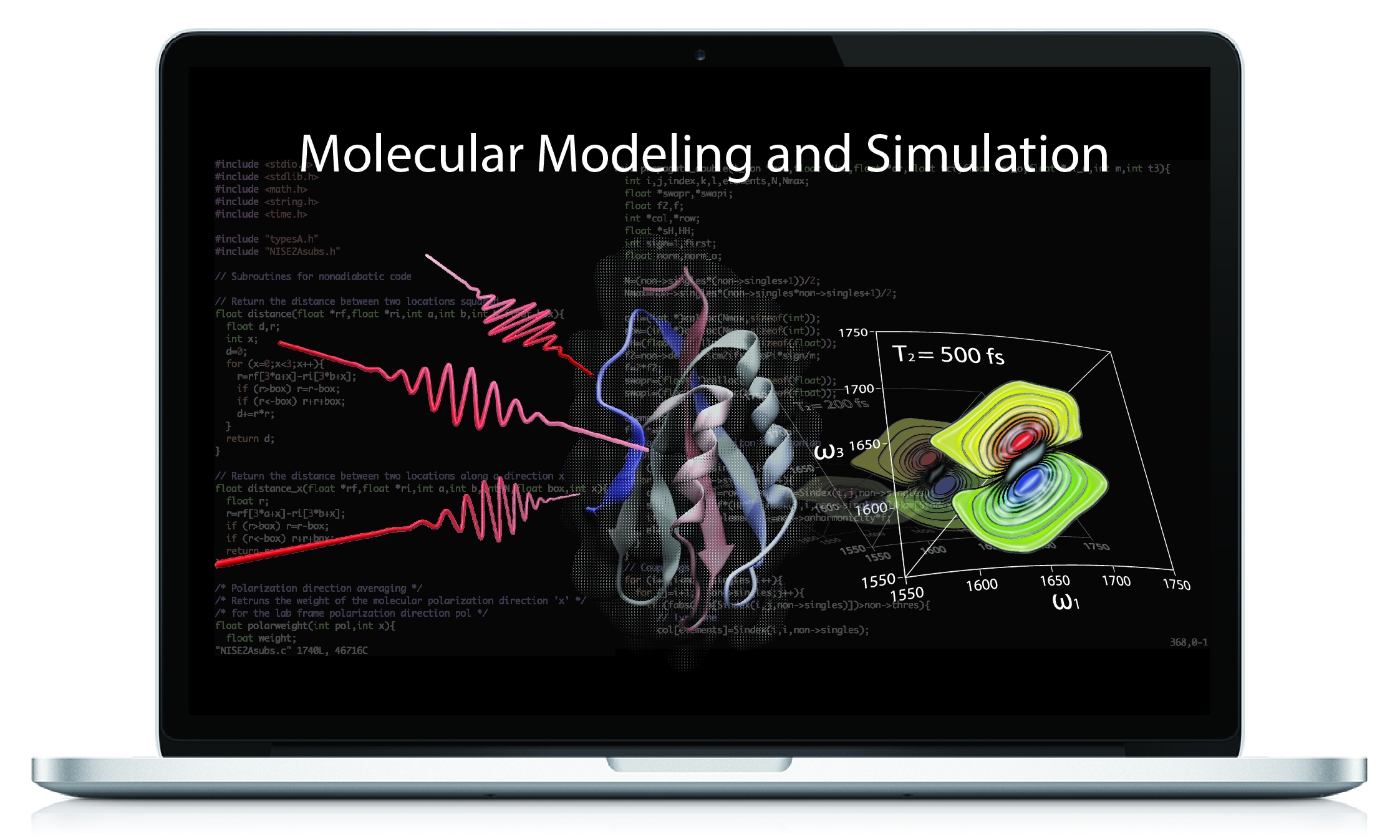
Molecular Modeling and Simulation Using Computational Approaches
Molecular modeling and simulation provide crucial insights into the structure, dynamics, and interactions of various systems at the molecular level, to both complement and enrich experimental measurements. This lab module training will introduce basic theories of popular modeling and simulation approaches, including molecular visualization, molecular dynamics simulation, quantum chemistry calculation, and molecular docking. The training also provides hands-on tutorials of widely-used software packages.
Instructor: Chungwen Liang (Director, IALS Computational Modeling Core)
Introduction to Synthetic Biology
Synthetic biology involves the design and construction of biological parts and systems for useful proposes. This lab module will cover basic concepts as well as provide hands on experience using common techniques in the field. Students will learn how to assemble DNA, starting with how to design and create PRC products which will be used as parts ina type IIs assembly. Once DNA is assembled it will be introduced into E. coli and students will be shown how to use flow cytometry to analyze the fluorescent output of the modified cells.
Instructors: Dr. Lauren Andrews and Matthew Lebovich, PhD Candidate (Chemical Engineering)
Fundamentals of AMNIS: Imaging Flow Cytometry
AMNIS yields quantitative population data in combination with fluorescent images of the cells. The applications of AMNIS are endless and can be applied across disciplines including polymer delivery, autophagy, cell division, apoptosis, and protein colocalization. This module will teach participants the basics of sample preparation, running samples, and data analysis.
Introduction to Biomaterials and their Applications
This lab module will combine hands-on activities in chemical synthesis, hydrogel network polymerization, mechanical characterization, cell culture, and microscopy to create biomaterials for applications in cell culture. The module will define and familiarize students with the term “biomaterial,” discuss the application of biomaterials, and provide hands-on experience working with synthetic polymer hydrogels for tissue engineering. Instructors will teach methods for creating novel monomers to include in biomaterials, growing cells on these materials, and chemically and functionally characterizing them. The module is designed for those with little to no familiarity with biomaterials.
Instructors: Profs. Shelly Peyton (Chemical Engineering) and Todd Emrick (Polymer Science and Engineering)


This module will expose students to optics theory and experiment by building a transmitted light microscope. Lecture will include basic ray optics. Hands on work will include optics basics culminating in the construction of a light microscope, leading to the imaging of several specimens.
Instructor: Prof. Jennifer Ross (Physics)


Topics on Fluorescence Spectroscopy: Fluorescence Lifetime Determinations
The fluorescence lifetime is one of the most important characteristics of a fluorescent probe because it defines the time window of observation of dynamic phenomena (FRET, quenching, anisotropy). We will introduce the principles for pulse fluorometry and phase-modulation fluorometry, and learn to determine the lifetime of fluorophores in biological samples.
Instructor: Prof. Alejandro Heuck (BMB/MCB)


 The biotech community at UMass has a decade-long tradition that started as part of the Institute Cellular Engineering to teach laboratory modules on cutting edge research techniques.
The biotech community at UMass has a decade-long tradition that started as part of the Institute Cellular Engineering to teach laboratory modules on cutting edge research techniques.








