Research Summary
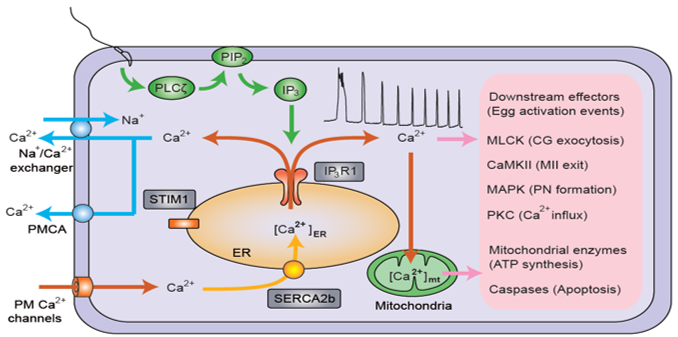
Egg activation is the first stage of embryo development and is induced by the sperm upon fusing with a mature oocyte, henceforth referred to as egg. In mammals, egg activation is triggered by a series of repeated changes in the intracellular concentration of free calcium (Ca2+), which are known as Ca2+ oscillations; a sperm-specific enzyme, phospholipase C zeta 1 (PLC ζ), is thought to trigger the Ca2+ oscillations. Although the first Ca2+ rises represent Ca2+ release from intracellular stores, influx of Ca2+ from the surrounding environment is necessary to maintain the persistent oscillations that can last in excess of 6 hours. There are several channels proposed to mediate the influx of extracellular Ca2+ in mouse oocytes and eggs such as voltage activated calcium channels (CaV), calcium release activated current channel (CRAC), and transient receptor potential family of channels (TRPs). Thus far, generation of CaV3.2 and CRAC channel knock (KO) strains does not affect fertility suggesting that these channels are not essential for fertilization and embryo development. The TRP family consist of ~30 members grouped into six subfamilies. TRPV3 mediates Ca2+ influx in mouse oocytes but TRPV3 knockout mice are fertile. Importantly, global TRPM7 KO embryos are unable to proceed beyond embryonic day 7.5, highlighting the importance of this channel for egg activation and/or early embryo development. Nevertheless, the protein expression of this channel in oocytes, eggs and during early embryonic stages in mammals is presently unknown. Further, the mechanism(s) whereby the lack of TRMP7 affects embryo development is also unknown.
Our laboratory has discovered the expression of both TRPV3 and TRPM7 channels in mouse oocytes and eggs. Given the requirement of TRPM7 for embryo development, my thesis will focus on TRPM7. Using electrophysiology, we demonstrated that TRPM7 mediates Ca2+ influx in oocytes and eggs. We also showed that specific pharmacological inhibition of the channel delayed pre-implantation embryo development and reduced progression to the blastocyst stage; these studies revealed that embryonic cleavage slows down and ceases around the 8-32 cells stage, which is significantly earlier than E7.5 noted in the previous global KO studies.
The primary goal of this project is to determine the pattern and level of expression of this channel in oocytes, eggs and pre-implantation embryos using immunofluorescence and western blot studies. Another goal, is to use the oocyte- and sperm-specific conditional KO TRPM7 (cKO) lines already available in our laboratory and specific nuclear stains to establish the precise stage of embryo development at which cleavage slows down and ceases in embryos devoid of TRPM7. Once we establish the timing of embryonic arrest, we plan to perform RNA-seq in both cKOs and wildtype to establish the cellular pathway(s) that is/are regulated by TRPM7. Future studies will determine whether the channel or the kinase portion or both domains of this chanzyme are important for the phenotype, and what divalent cation is most implicated in the arrest of embryonic development. Characterisation of the expression and regulation of TRPM7 in gametes and embryos will make possible the optimization of Mg2+ concentration in commercial culture media, aiding embryo development and success in IVF clinics. TRPM7 may also serve as a target to develop non-hormonal contraception approaches.